Topical Calcineurin Inhibitors: Effective Eczema Treatment
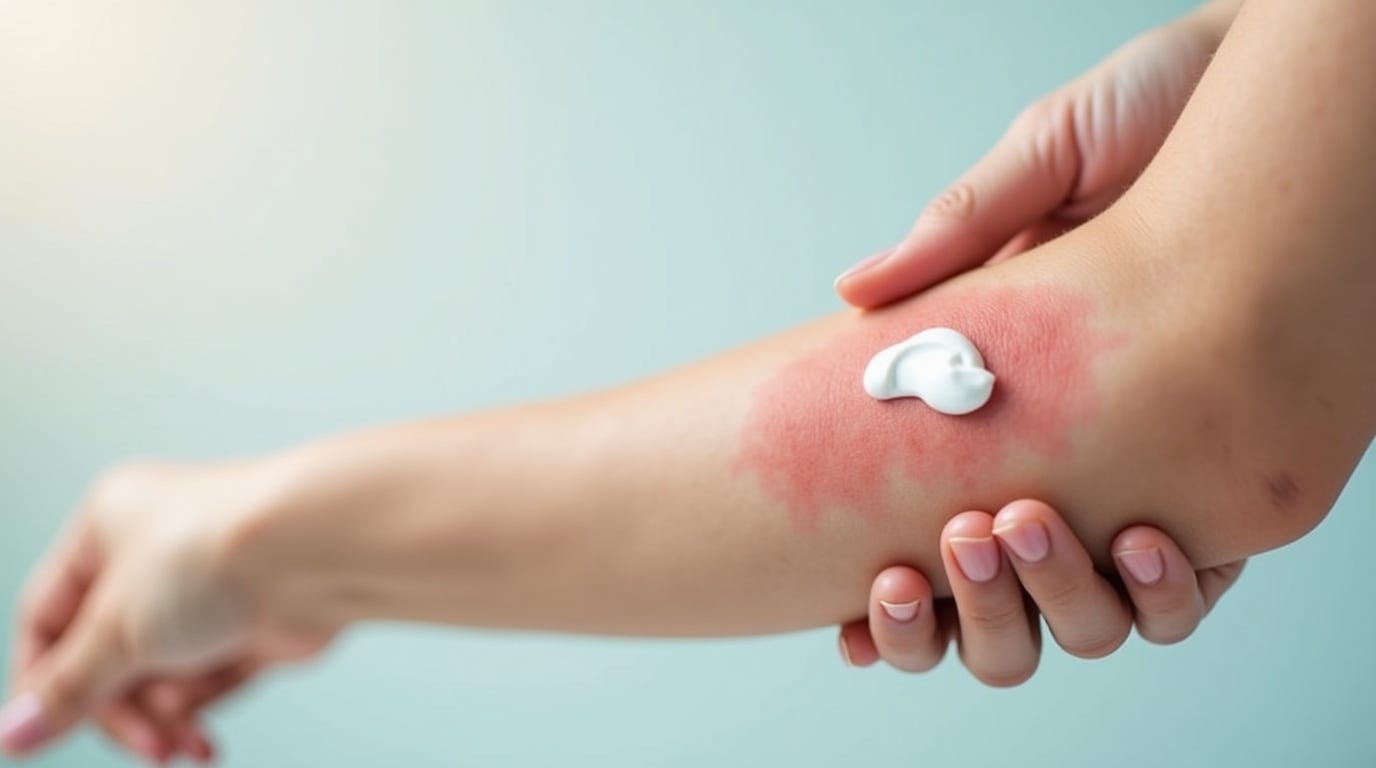
Topical Calcineurin Inhibitors (TCIs) represent one of the most significant advancements in dermatological treatments over the past two decades. These medications offer an effective non-steroidal alternative for managing various inflammatory skin conditions, particularly atopic dermatitis. With their unique mechanism of action that targets specific inflammatory pathways, TCIs have revolutionized treatment options for millions of patients worldwide who struggle with chronic skin conditions. This comprehensive guide explores the science behind TCIs, their applications, benefits, safety profile, and practical usage recommendations to help you better understand this important class of medications.
Understanding Calcineurin and Its Role in Skin Inflammation
The Science of Skin Inflammation
Calcineurin is a protein enzyme that plays a crucial role in activating the immune system's inflammatory response. In normal immune function, calcineurin serves as a signaling molecule that helps regulate T-cell activation. T-cells are white blood cells that act as essential soldiers in your body's defense system, producing various inflammatory mediators when they encounter potential threats. However, in conditions like atopic dermatitis (eczema), this system becomes dysregulated, leading to excessive inflammation in the skin.
The immune system of individuals with inflammatory skin conditions like atopic dermatitis tends to overreact to certain environmental irritants and allergens. When triggered, calcineurin activates genes that produce inflammatory cytokines – messenger molecules that perpetuate inflammation and cause symptoms like redness, swelling, and itching. This overactive immune response creates the characteristic inflammation seen in many dermatological conditions.
How Calcineurin Inhibition Works
Topical calcineurin inhibitors function by binding to specific proteins in skin cells called macrophilin-12 or FK506-binding protein (FKBP). When TCIs bind to these proteins, they form a complex that prevents calcineurin from activating nuclear factor of activated T-cells (NFAT), effectively blocking the cascade of inflammatory signals. This targeted approach helps reduce inflammation at its source without affecting other bodily systems significantly.
By interfering with this specific pathway, TCIs can effectively suppress the production of various pro-inflammatory cytokines including interleukins (IL-2, IL-3, IL-4, IL-5), interferon-gamma, and tumor necrosis factor-alpha. These cytokines are key drivers of skin inflammation in conditions like atopic dermatitis. This focused mechanism allows TCIs to provide significant anti-inflammatory effects while potentially avoiding some of the side effects associated with broader-acting treatments.
Types of Topical Calcineurin Inhibitors
Main Types and Formulations
There are two primary types of topical calcineurin inhibitors currently available for dermatological use. The first type comes in an ointment form at concentrations of 0.03% and 0.1%, with different strengths appropriate for different age groups and severity levels. The second type is available as a 1% cream formulation. These medications have been used systemically for immunosuppression in transplantation medicine at approximately 1,000 times higher doses than their topical formulations, highlighting their safety when used topically as directed.
The ointment formulation is more occlusive and may provide better penetration for drier, thicker skin lesions. This formulation is generally recommended for moderate to severe eczema. The cream formulation has a lighter feel and is typically preferred for mild to moderate cases. The choice between these formulations often depends on the severity of the condition, the affected body area, and patient preference regarding the feel of the medication.
Strength Variations and Appropriate Usage
The ointment formula comes in two strengths: 0.03% for milder cases or for children aged 2 to 16 years, and 0.1% for moderate to severe cases in adults and adolescents 16 years and older. The cream formulation is available as a 1% preparation and is indicated for mild to moderate atopic dermatitis in patients as young as 3 months of age.
These different formulations and strengths provide clinicians with flexibility to tailor treatment to individual patients based on their age, severity of condition, and the specific body regions affected. This personalized approach helps optimize efficacy while minimizing potential side effects, making TCIs a valuable tool in the dermatologist's arsenal.
Mechanism of Action: How TCIs Work in the Skin
Cellular Effects of Calcineurin Inhibition
Topical calcineurin inhibitors work through a well-defined molecular mechanism. When applied to the skin, these medications penetrate the epidermis and dermis where they interact with immune cells. Inside these cells, TCIs bind to specific cytoplasmic proteins called immunophilins. This binding creates a complex that specifically inhibits the activity of calcineurin.
Calcineurin typically functions by dephosphorylating the nuclear factor of activated T-cells (NFAT), allowing it to enter the cell nucleus and initiate transcription of genes that produce inflammatory cytokines. By blocking this process, TCIs effectively prevent the production of various pro-inflammatory substances that would otherwise trigger and sustain skin inflammation.
Differences from Corticosteroid Mechanism
Unlike topical corticosteroids which have broad anti-inflammatory effects across multiple pathways, TCIs work through a more targeted mechanism. Corticosteroids affect numerous aspects of the inflammatory process, which explains both their potent efficacy and their potential for more widespread side effects. TCIs, by contrast, specifically target the calcineurin-dependent activation of T-cells, providing a more focused approach to controlling inflammation.
This targeted mechanism offers significant advantages, particularly for long-term management and treatment of sensitive areas. Most notably, TCIs do not cause skin atrophy (thinning), telangiectasia (visible blood vessels), or striae (stretch marks) – all common side effects associated with prolonged corticosteroid use. This makes TCIs particularly valuable for treating inflammatory conditions on the face, eyelids, genitals, and skin folds where the skin is naturally thinner and more vulnerable to steroid-related side effects.
Medical Indications and Approved Uses
Primary Indication: Atopic Dermatitis
The primary and officially approved indication for topical calcineurin inhibitors is atopic dermatitis, commonly known as eczema. Atopic dermatitis is a chronic, relapsing inflammatory skin condition characterized by intense itching, dryness, and red, inflamed skin. TCIs have been extensively studied in this condition and have demonstrated significant efficacy in reducing inflammation, relieving itching, and improving overall skin appearance.
For atopic dermatitis, TCIs can be used in several ways: as short-term treatment for active flares, as maintenance therapy to prevent recurrences, and as targeted treatment for particularly troublesome areas like the face, neck, and skin folds. The ointment formulation is typically recommended for moderate to severe atopic dermatitis, while the cream formulation is used for mild to moderate cases.
Off-Label Uses for Other Skin Conditions
While atopic dermatitis remains the only officially approved indication, dermatologists frequently prescribe TCIs for several other inflammatory skin conditions where they have shown efficacy in clinical studies. These off-label uses include seborrheic dermatitis (especially on the face), contact dermatitis, psoriasis (particularly on the face and intertriginous areas), lichen sclerosus (especially genital variants), oral lichen planus, vitiligo, and alopecia areata.
TCIs are particularly valuable in these conditions when they affect sensitive areas where topical corticosteroids might cause significant side effects. The ability of TCIs to provide anti-inflammatory benefits without causing skin atrophy makes them especially useful for facial rashes, genital inflammation, and conditions affecting skin folds.
Age-Specific Recommendations
There are specific age recommendations for the different formulations of TCIs. The 0.1% ointment formulation is approved for adults and adolescents aged 16 and older with moderate to severe atopic dermatitis. The 0.03% ointment is approved for children aged 2 years and older. The cream formulation is indicated for mild to moderate atopic dermatitis in patients aged 3 months and older.
These age restrictions are based on clinical studies evaluating safety and efficacy in different age groups. It's worth noting that many dermatologists consider TCIs particularly valuable for treating facial eczema in infants and young children, where corticosteroids carry higher risks of side effects. However, they must always be used under medical supervision and according to approved guidelines.
Efficacy of Topical Calcineurin Inhibitors
Clinical Evidence of Effectiveness
Numerous clinical trials have established the efficacy of topical calcineurin inhibitors in treating atopic dermatitis. These studies consistently show that TCIs significantly reduce disease severity, improve symptoms like itching and sleep disturbance, and enhance quality of life for patients with atopic dermatitis. Research indicates that the anti-inflammatory effects of TCIs are comparable to medium-potency topical corticosteroids, making them effective alternatives when corticosteroids are contraindicated or causing adverse effects.
In terms of onset of action, improvement is typically noticeable within the first week of treatment, with significant improvement often achieved by weeks 3-6 of consistent use. This relatively rapid response makes TCIs suitable for managing acute flares as well as for maintenance therapy to prevent recurrences.
Comparative Efficacy Between Different Formulations
Studies comparing the two main types of TCIs have found notable differences in their efficacy profiles. Meta-analyses indicate that the ointment formulation tends to have higher efficacy than the cream formulation in treating atopic dermatitis, particularly for moderate to severe cases in both adults and children. Specifically, research has demonstrated that the 0.1% ointment formulation is more effective than the 1% cream formulation in adult patients and in moderate to very severe pediatric patients.
The ointment formulation (0.03%) and cream formulation (1%) appear to have similar efficacy in treating mild to moderate atopic dermatitis in children. This information helps clinicians select the most appropriate formulation based on patient age, disease severity, and affected body regions.
Long-Term Efficacy and Maintenance Therapy
TCIs have demonstrated excellent efficacy in long-term management strategies for atopic dermatitis. Studies show that intermittent, proactive application of TCIs can significantly reduce the frequency and severity of disease flares compared to reactive treatment approaches. This maintenance approach typically involves applying the medication to previously affected areas twice weekly even after the active inflammation has resolved.
This proactive strategy addresses the chronic, relapsing nature of atopic dermatitis by targeting subclinical inflammation that may be present even when the skin appears normal. By maintaining control of this low-grade inflammation, TCIs can help break the cycle of frequent flares that is characteristic of chronic atopic dermatitis, providing better long-term disease control and improved quality of life for patients.
Application and Usage Guidelines
Proper Application Techniques
The effectiveness of topical calcineurin inhibitors depends significantly on proper application. These medications should be applied to clean, dry skin in a thin layer, covering only the affected areas. Unlike some other topical medications, TCIs should not be applied under occlusion (covered with plastic wrap or waterproof bandages) unless specifically directed by a healthcare provider, as this can increase absorption and potentially increase side effects.
For optimal results, patients should wash and gently dry the affected skin before application. A small amount of the medication should be applied and rubbed in gently until it disappears into the skin. It's important to wash hands after application unless the hands themselves are being treated. This helps prevent accidental transfer of the medication to other areas, particularly the eyes or mouth.
Frequency and Duration Recommendations
For active flares of atopic dermatitis, TCIs are typically applied twice daily-once in the morning and once in the evening-until the inflammation resolves. Improvement is usually seen within a few days, with significant improvement within 1-3 weeks. Once the acute symptoms resolve, different approaches to maintenance therapy may be recommended.
For the ointment formulation, application twice daily for up to 3 weeks followed by once-daily application until symptom resolution is a common regimen for patients aged 2-16 years. For adults using the 0.1% formulation, twice-daily application until symptoms resolve is typically recommended. For the cream formulation, application twice daily for up to 6 weeks is the standard recommendation.
Special Considerations for Sensitive Areas
One of the most valuable aspects of TCIs is their suitability for use on sensitive areas such as the face, neck, eyelids, and skin folds where the skin is naturally thinner and more permeable. These areas are particularly vulnerable to side effects from topical corticosteroids, such as skin atrophy and telangiectasia. TCIs do not cause these structural changes to the skin, making them ideal for these sensitive locations.
For genital areas, TCIs can be especially beneficial due to their favorable safety profile. However, it's normal to experience some burning or irritation when applying TCIs to inflamed skin in sensitive regions. Patients should be advised that this sensation typically diminishes with continued use as the skin inflammation improves.
Safety Profile and Potential Side Effects
Common Side Effects
The most commonly reported side effects of topical calcineurin inhibitors are localized sensations at the application site. These include burning, stinging, itching, and redness, which typically occur during the first few days of treatment and diminish over time as the skin inflammation improves. These application site reactions are generally mild to moderate and rarely lead to discontinuation of treatment.
Interestingly, these sensations are more common with the ointment formulation than with the cream formulation, particularly in adult patients. However, research indicates that despite this initial discomfort, fewer patients withdraw from treatment with TCIs due to adverse events compared to other topical treatments, suggesting good overall tolerability.
FDA Warning and Malignancy Concerns
In 2006, the U.S. Food and Drug Administration (FDA) added a "black box" warning to TCIs regarding a theoretical risk of malignancy, particularly skin cancer and lymphoma. This warning was based primarily on three factors: high-dose animal studies, case reports of cancer in patients using TCIs, and the known effects of systemic (oral or injected) calcineurin inhibitors used at much higher doses in organ transplant patients.
However, it's important to understand that numerous long-term studies and analyses conducted since then have not demonstrated a causal relationship between topical calcineurin inhibitors and increased cancer risk when used as directed. The amount of medication absorbed through the skin with topical application is minimal-approximately 1/1000th of the doses used systemically for organ transplantation. Nevertheless, as a precaution, it's recommended to use sun protection when using TCIs and to apply the medication in the evening when possible.
Long-Term Safety Considerations
Extensive research into the long-term safety of TCIs has been reassuring. Unlike topical corticosteroids, TCIs do not cause skin atrophy, striae (stretch marks), or telangiectasia (visible blood vessels) even with prolonged use. This makes them particularly valuable for long-term management of chronic skin conditions.
With regard to immune function, studies have not shown clinically significant systemic immunosuppression with topical use as directed. Local immunosuppression at the site of application may occur, but this is actually part of the therapeutic effect in treating inflammatory skin conditions. However, there may be a slightly increased risk of localized skin infections or herpes virus reactivation in some patients, highlighting the importance of monitoring for signs of infection during treatment.
Special Populations and Considerations
Use in Children
TCIs are valuable treatment options for children with atopic dermatitis, particularly for sensitive areas like the face, neck, and skin folds where corticosteroids might cause problematic side effects. The ointment formulation at 0.03% concentration is approved for children aged 2 years and older, while the cream formulation is approved for children as young as 3 months.
Although TCIs are not officially approved for children under these age limits, many dermatologists consider them particularly valuable for treating facial eczema in infants, where the risks of corticosteroid side effects are higher. However, the decision to use TCIs in very young children should always be made by a specialist after carefully considering the potential benefits and risks for each individual patient.
Pregnancy and Breastfeeding Considerations
The safety of TCIs during pregnancy and breastfeeding has not been extensively studied in humans. Animal studies have not shown evidence of harm to the fetus, but due to limited human data, TCIs are typically used with caution during pregnancy and only when the potential benefits outweigh the potential risks.
Regarding breastfeeding, small amounts of systemically administered calcineurin inhibitors can be detected in breast milk. However, the minimal absorption from topical application suggests that the amount in breast milk would be negligible with topical use. Nevertheless, if TCIs are used while breastfeeding, it's advisable to avoid applying the medication to the breast area, and to ensure it's fully absorbed before breastfeeding to minimize infant exposure.
Interactions with Vaccinations
Because TCIs have immunomodulatory effects, they could theoretically affect the response to vaccines, particularly at the site of application. As a general precaution, it's recommended to avoid applying TCIs to vaccination sites for at least 14 days after vaccination. For patients requiring extensive treatment with TCIs, it may be prudent to discuss vaccination timing with healthcare providers to ensure optimal vaccine efficacy.
For live vaccines in particular, if a patient has extensive skin disease requiring large surface area treatment with TCIs, it might be advisable to temporarily discontinue the TCI or to carefully time the vaccination during a period of better disease control requiring less medication use. These decisions should be made in consultation with the patient's dermatologist and immunization provider.
Comparing TCIs with Other Treatment Options
TCIs vs. Topical Corticosteroids
Topical corticosteroids (TCS) have long been the first-line treatment for inflammatory skin conditions, particularly atopic dermatitis. They work quickly and effectively, but long-term use carries risks of local side effects including skin atrophy, telangiectasia, striae, and pigment changes. In contrast, TCIs do not cause these structural changes to the skin, making them particularly valuable for sensitive areas and long-term use.
In terms of efficacy, medium-potency topical corticosteroids generally act more quickly and effectively than TCIs for acute flares. However, TCIs offer a safer alternative for long-term management, sensitive areas, and maintenance therapy. The anti-inflammatory potency of the ointment formulation is similar to a moderate-potency corticosteroid, while the cream formulation has somewhat lower potency.
Integrating TCIs into a Treatment Regimen
TCIs can be effectively integrated into a comprehensive treatment approach for atopic dermatitis and other inflammatory skin conditions. They can be used in several ways:
-
As alternatives to topical corticosteroids for sensitive areas like the face, neck, and skin folds
-
As steroid-sparing agents to reduce the long-term exposure to corticosteroids
-
In rotation with topical corticosteroids to minimize side effects from either medication
-
As maintenance therapy to prevent disease recurrence after control with corticosteroids
-
As first-line therapy for mild to moderate disease, particularly in sensitive areas
This flexible approach allows dermatologists to tailor treatment to each patient's specific needs, taking into account factors like disease severity, affected body regions, age, and response to previous treatments.
Cost-Effectiveness Considerations
Cost is an important consideration when choosing between TCIs and other treatment options. Generally, TCIs tend to be more expensive than generic topical corticosteroids, which may affect accessibility for some patients. However, when considering the total cost of care, including the management of potential side effects from long-term corticosteroid use, TCIs may offer good value, particularly for maintenance therapy and treatment of sensitive areas.
Limited studies comparing cost-effectiveness between the two main types of TCIs suggest that the 0.1% ointment formulation might yield better clinical outcomes and lower costs of care than the 1.0% cream formulation in adults with atopic dermatitis. However, more comprehensive economic analyses are needed to fully understand the comparative value of different treatment approaches.
Practical Tips for Patients Using TCIs
Managing Application Site Reactions
Many patients experience burning, stinging, or itching sensations when first applying TCIs, particularly to inflamed skin. These sensations typically diminish over time as the skin condition improves. Several strategies can help manage these reactions:
-
Applying the medication to cool, dry skin (rather than warm, damp skin)
-
Starting with a smaller amount and gradually increasing as tolerated
-
Considering brief pre-treatment with a topical anesthetic if reactions are severe (under medical supervision)
-
Refrigerating the medication before application (though this should be discussed with a healthcare provider first)
-
Persisting with treatment, as these sensations typically diminish within a week of regular use
For many patients, the temporary discomfort is outweighed by the long-term benefits and safety advantages of TCIs compared to other treatment options.
Maximizing Treatment Effectiveness
To get the most benefit from TCI treatment, patients should follow these practical guidelines:
-
Apply TCIs to clean, dry skin for optimal absorption
-
Use only the amount needed to cover the affected area thinly
-
Follow the prescribed frequency and duration of application
-
Continue treatment until the skin is clear, not just until symptoms improve
-
For maintenance therapy, apply the medication to previously affected areas even when the skin appears normal
-
Combine with gentle skin care, including regular moisturizing and avoiding known irritants
-
Use sun protection when treating exposed areas, particularly during daylight hours
Consistency in application and integration with a comprehensive skin care routine significantly improves outcomes with TCI treatment.
Recognizing When to Seek Further Medical Advice
While TCIs are generally safe and effective, patients should know when to consult their healthcare provider about their treatment. Signs that warrant medical attention include:
-
Skin infections at the treatment site (increased redness, warmth, swelling, or yellow crust)
-
Worsening of the skin condition despite consistent treatment
-
Severe or persistent application site reactions that don't improve with continued use
-
Development of new or unexpected symptoms
-
Signs of systemic absorption (extremely rare but could include fatigue, headache, or nausea)
Regular follow-up visits with healthcare providers are important for patients using TCIs long-term to monitor treatment effectiveness and detect any potential issues early.
Future Developments in Calcineurin Inhibitor Treatments
Emerging Research and New Formulations
Research into topical calcineurin inhibitors continues to evolve, with several promising developments on the horizon. Scientists are exploring new formulations that might offer improved efficacy, better tolerability, or expanded indications. These include novel vehicle formulations designed to enhance penetration while minimizing application site reactions, combination products that incorporate TCIs with other anti-inflammatory agents, and modified release systems for prolonged action.
Additionally, researchers are investigating the potential use of TCIs for a wider range of dermatological conditions beyond atopic dermatitis. Preliminary studies suggest efficacy in conditions like psoriasis, vitiligo, lichen planus, and other inflammatory dermatoses, potentially expanding the therapeutic utility of these medications in the future.
Evolving Understanding of Long-term Outcomes
As TCIs have now been in clinical use for over two decades, researchers have accumulated substantial data on long-term outcomes. Ongoing longitudinal studies are helping to further clarify the long-term safety profile, particularly regarding the theoretical concerns about malignancy risk. So far, these studies have been reassuring, showing no significant increase in cancer risk with topical use as directed.
Future research will continue to monitor these outcomes and refine our understanding of optimal use patterns, potentially leading to revised guidelines for TCI use in various patient populations. This evolving evidence base will help clinicians and patients make more informed decisions about incorporating TCIs into long-term management strategies for chronic skin conditions.
Conclusion
Topical calcineurin inhibitors represent a significant advancement in the treatment of inflammatory skin conditions, particularly atopic dermatitis. With their unique mechanism of action targeting specific inflammatory pathways, TCIs offer an effective alternative to topical corticosteroids, especially for sensitive areas and long-term management. They have demonstrated significant efficacy in reducing inflammation, relieving symptoms, and improving quality of life for patients with chronic skin conditions.
The two main formulations-ointment and cream-provide options for different severity levels, age groups, and body regions. While the ointment formulation generally offers greater efficacy for moderate to severe cases, the cream formulation provides a lighter alternative for mild to moderate conditions. Both have valuable roles in comprehensive dermatological care.
Despite initial concerns about potential malignancy risks, extensive research and clinical experience have provided reassurance about the safety of TCIs when used as directed. The most common side effects are temporary application site reactions that typically diminish with continued use. The lack of skin atrophy and other structural changes makes TCIs particularly valuable for treating sensitive areas and for long-term management strategies.
As research continues to evolve, our understanding of optimal use patterns and potential new applications for TCIs will continue to expand. For now, these medications remain an essential component of the dermatological armamentarium, offering significant benefits for patients with atopic dermatitis and other inflammatory skin conditions.
Frequently Asked Questions about Topical Calcineurin Inhibitors
1. What exactly are topical calcineurin inhibitors and how do they differ from corticosteroids?
Topical calcineurin inhibitors (TCIs) are non-steroidal anti-inflammatory medications applied to the skin to treat conditions like atopic dermatitis (eczema). Unlike corticosteroids that affect multiple inflammatory pathways, TCIs work by specifically blocking calcineurin, a protein that activates T-cells in the immune system. This targeted approach reduces inflammation without causing skin thinning (atrophy), stretch marks, or visible blood vessels that can occur with long-term corticosteroid use. TCIs are particularly valuable for sensitive areas like the face, neck, and skin folds where corticosteroids might cause problematic side effects. They come in ointment and cream formulations, with the ointment generally being more potent and suitable for moderate-to-severe conditions, while the cream works well for mild-to-moderate cases. Both formulations are effective alternatives when corticosteroids are contraindicated or causing adverse effects.
2. How long does it take for topical calcineurin inhibitors to show results?
Most patients begin noticing improvement within the first week of consistently using topical calcineurin inhibitors. Significant improvement typically occurs within 3-6 weeks of regular, twice-daily application. The ointment formulation generally works slightly faster than the cream formulation, particularly for moderate-to-severe cases. Initial response often includes reduction in itching and burning sensations, followed by visible improvement in redness and inflammation. For some patients, particularly those with long-standing or severe eczema, it may take longer to see maximum benefit. It's important to continue using the medication as prescribed even after symptoms begin to improve, as premature discontinuation can lead to rapid relapse. For maintenance therapy, regular application (typically twice weekly) to previously affected areas can significantly reduce the frequency and severity of flares, with this preventive effect typically established after several weeks of consistent use.
3. Is it true that TCIs increase cancer risk? Should I be concerned?
The concern about cancer risk stems from a 2006 FDA "black box" warning based on animal studies using high doses, case reports, and the known effects of oral calcineurin inhibitors used at much higher doses in organ transplant patients. However, numerous long-term studies conducted since then have not demonstrated a causal relationship between topical calcineurin inhibitors and increased cancer risk when used as directed. The amount absorbed through the skin with topical application is minimal-approximately 1/1000th of the doses used systemically for transplantation. Major dermatology organizations worldwide have questioned the validity of this warning based on accumulated evidence. Nevertheless, reasonable precautions include using sun protection when using TCIs on exposed skin, applying TCIs in the evening when possible, and following your healthcare provider's recommendations regarding appropriate use. Your dermatologist can provide personalized guidance based on your specific situation and help you weigh the well-established benefits against theoretical risks.
4. Can topical calcineurin inhibitors be used alongside other treatments for eczema?
Yes, topical calcineurin inhibitors can be effectively combined with other treatments for eczema as part of a comprehensive management approach. They work particularly well in rotation with topical corticosteroids, allowing patients to minimize steroid exposure while maintaining disease control. A common strategy is to use corticosteroids for acute flares, then transition to TCIs for maintenance. TCIs can also be safely used alongside moisturizers, which should be applied first, with TCIs applied after the moisturizer has been absorbed. They're compatible with phototherapy treatments, though it's best to apply TCIs after phototherapy sessions rather than before. TCIs can also be used with systemic medications for severe eczema, including antihistamines, oral anti-inflammatory agents, and biologics. For wet wraps, TCIs should be applied before the wet dressing. Always follow your healthcare provider's specific instructions regarding timing and sequence when combining multiple treatments to ensure optimal effectiveness and safety.
5. Are topical calcineurin inhibitors safe for children and infants?
Topical calcineurin inhibitors have established safety profiles for children, with specific age recommendations for different formulations. The ointment formulation at 0.03% concentration is approved for children aged 2 years and older, while the cream formulation is approved for children as young as 3 months. For children, TCIs offer several advantages over topical corticosteroids, particularly for sensitive areas like the face and neck, as they don't cause skin thinning or growth suppression. The most common side effects in children are temporary burning or stinging sensations at the application site that typically diminish with continued use. While TCIs carry an FDA warning regarding theoretical malignancy risk, long-term studies in pediatric populations have not demonstrated increased cancer rates. Children using TCIs should practice sun protection, and treatment should be supervised by a healthcare provider. For very young children or infants below approved age thresholds, treatment decisions should be made by specialists after carefully weighing potential benefits against theoretical risks.
6. How should I apply topical calcineurin inhibitors for maximum effectiveness?
For maximum effectiveness, apply topical calcineurin inhibitors to clean, dry skin in a thin layer covering only affected areas. Begin by washing the treatment area with a gentle, fragrance-free cleanser and patting (not rubbing) the skin dry. Wait about 15-20 minutes for the skin to be completely dry before application, as moist skin can increase absorption and potential irritation. Use only the amount needed to cover the affected area thinly-using more doesn't increase effectiveness but may increase side effects. Gently rub the medication into the skin until it disappears. Wash your hands after application unless treating hand eczema. For twice-daily regimens, space applications approximately 12 hours apart. Don't cover treated areas with occlusive dressings unless specifically directed by your healthcare provider. Continue treatment until the skin is completely clear, not just until symptoms improve. Consistency is key-missing applications reduces effectiveness. For maintenance therapy, follow your provider's recommendations for frequency, typically twice weekly application to previously affected areas even when skin appears normal.
7. What side effects should I expect when using TCIs and how can I manage them?
The most common side effects of topical calcineurin inhibitors are temporary sensations at the application site, including burning, stinging, itching, and warmth. These reactions occur in approximately 20-60% of patients, particularly during initial treatment when the skin is inflamed, and typically diminish within 3-7 days of continued use. The ointment formulation tends to cause more pronounced sensations than the cream formulation. To manage these reactions, try applying the medication to cool, dry skin, starting with smaller amounts, or refrigerating the medication before application (with provider approval). Avoiding application immediately after bathing when pores are open may help. Brief pre-treatment with ice can reduce discomfort in sensitive individuals. Less common side effects include temporary redness or mild headache. Rare side effects include folliculitis or increased risk of skin infections. Contact your healthcare provider if you experience severe or persistent burning, signs of skin infection (increased redness, warmth, swelling, or yellow crust), or worsening of your condition despite consistent treatment.
8. Can topical calcineurin inhibitors be used during pregnancy or while breastfeeding?
The safety of topical calcineurin inhibitors during pregnancy hasn't been thoroughly studied in humans. Animal studies haven't shown evidence of harm to the fetus, but due to limited human data, TCIs are typically used with caution during pregnancy and only when potential benefits outweigh potential risks. If you're pregnant or planning to become pregnant, discuss the benefits and risks with your healthcare provider. Alternative treatments with longer safety records during pregnancy may be considered first. Regarding breastfeeding, small amounts of systemically administered calcineurin inhibitors can be detected in breast milk. However, the minimal absorption from topical application suggests that the amount in breast milk would be negligible with topical use. If using TCIs while breastfeeding, avoid applying the medication to the breast area, ensure it's fully absorbed before breastfeeding, and consider temporarily wiping the application area before direct skin-to-skin contact with your baby. Always inform your dermatologist and obstetrician about all medications you're using during pregnancy or breastfeeding.
9. Can TCIs be used for skin conditions other than atopic dermatitis?
While the only FDA-approved indication for topical calcineurin inhibitors is atopic dermatitis, dermatologists frequently prescribe them off-label for several other inflammatory skin conditions where they've shown efficacy in clinical studies. These conditions include seborrheic dermatitis (especially on the face), contact dermatitis, psoriasis (particularly on the face and intertriginous areas), lichen sclerosus (especially genital variants), oral and genital lichen planus, vitiligo, and discoid lupus erythematosus. TCIs are particularly valuable for these conditions when they affect sensitive areas where topical corticosteroids might cause significant side effects. For facial seborrheic dermatitis, TCIs can provide effective control without the risk of steroid-induced rosacea. In vitiligo, TCIs may help stimulate repigmentation, especially when combined with phototherapy. For autoimmune conditions like lichen planus, TCIs can help control inflammation without the atrophy risks associated with long-term steroid use. If you have a skin condition other than atopic dermatitis, consult a dermatologist about whether TCIs might be appropriate for your specific situation.
10. How long can I safely use topical calcineurin inhibitors?
Topical calcineurin inhibitors can be safely used for extended periods under medical supervision. Unlike topical corticosteroids, TCIs don't cause skin atrophy, striae, or telangiectasia even with prolonged use, making them suitable for long-term management strategies. For maintenance therapy, TCIs are often prescribed for twice-weekly application to previously affected areas to prevent flares, an approach that has been studied for up to 12 months with good safety profiles. Continuous daily use for acute management is typically limited to 3-6 weeks, after which patients should either discontinue treatment (if the condition has resolved) or transition to intermittent maintenance therapy. Long-term safety studies spanning several years have not shown evidence of cumulative toxicity or increasing side effects over time. Regular follow-up with your healthcare provider is important during extended treatment to monitor effectiveness and detect any potential issues. Your provider may periodically reassess your treatment plan, adjusting the frequency or concentration based on your response and changing needs.
11. How do the two main types of TCIs compare in terms of effectiveness and side effects?
The two main types of topical calcineurin inhibitors differ in their effectiveness and side effect profiles. Research indicates that the ointment formulation generally shows higher efficacy than the cream formulation, particularly for moderate to severe atopic dermatitis in both adults and children. The ointment formulation (0.1%) has demonstrated superior results compared to the cream formulation (1%) in controlled studies, with more patients achieving clear or almost clear skin. However, for mild to moderate cases in children, both formulations show similar effectiveness. Regarding side effects, the ointment formulation tends to cause more application site reactions (burning, stinging) than the cream formulation, particularly in adult patients. Despite this initial discomfort, fewer patients withdraw from ointment treatment due to lack of efficacy. The cream formulation has a lighter feel that some patients prefer, especially for facial application, but may require more frequent reapplication for optimal results. Your dermatologist will recommend the most appropriate formulation based on your age, the severity of your condition, affected body regions, and personal preferences.
12. Can I use TCIs on my face, eyelids, and other sensitive areas?
Yes, topical calcineurin inhibitors are particularly valuable for treating sensitive areas like the face, eyelids, neck, and skin folds where the skin is naturally thinner. Unlike topical corticosteroids, TCIs don't cause skin thinning, visible blood vessels, or other structural changes to the skin, making them especially suitable for these delicate regions. For facial application, TCIs are often preferred over corticosteroids because they don't cause steroid-induced rosacea or perioral dermatitis with long-term use. When applying TCIs around the eyes, use caution to avoid getting the medication directly in the eyes; if this occurs, rinse thoroughly with water. For genital areas, TCIs can be especially beneficial due to their favorable safety profile, though some burning sensation may initially occur. For skin folds (like underarms, groin, under breasts), where moisture can increase medication absorption, apply a thin layer and allow it to dry completely before covering the area. Always follow your healthcare provider's specific instructions regarding application to sensitive areas, as they may recommend adjusted frequency or concentration based on the specific region being treated.
13. Do I need to take special precautions regarding sun exposure when using TCIs?
Yes, special precautions regarding sun exposure are recommended when using topical calcineurin inhibitors. While the exact relationship between TCIs and photosensitivity is not fully established, prudent sun protection practices are advisable. These include applying broad-spectrum sunscreen with SPF 30 or higher to treated areas exposed to sunlight, wearing protective clothing like hats and long sleeves when outdoors, and limiting direct sun exposure during peak hours (10 AM to 4 PM). Some dermatologists recommend applying TCIs in the evening rather than the morning to minimize sun exposure to freshly treated skin. These recommendations stem partly from the FDA warning about theoretical malignancy risks and partly from the fact that many patients with inflammatory skin conditions have inherent photosensitivity. If you're using TCIs in combination with phototherapy treatments, follow your healthcare provider's specific instructions regarding timing-typically, phototherapy is performed first, followed by TCI application after an appropriate interval. These precautions don't mean you must avoid sunlight entirely, but rather practice sensible sun protection as part of your overall skin health management.
14. How do TCIs affect the immune system, and should I be concerned about increased infection risk?
Topical calcineurin inhibitors work by temporarily suppressing certain aspects of the local immune response in the skin, specifically targeting T-cell activation through calcineurin inhibition. This immune modulation occurs primarily at the application site with minimal systemic effects when used as directed. This localized impact means TCIs don't cause significant general immunosuppression like their oral counterparts used in transplant medicine. However, there is a slightly increased risk of localized skin infections, particularly viral infections like herpes simplex (cold sores) or molluscum contagiosum at treated sites. Studies suggest a small increase in these infections, though the absolute risk remains low. To minimize infection risk, avoid applying TCIs to obviously infected skin, maintain good hygiene practices, monitor for signs of infection (increased redness, warmth, swelling, or yellow crust), and promptly contact your healthcare provider if you notice these signs. People with significantly compromised immune systems should use TCIs under close medical supervision. For most patients, the infection risk is manageable and outweighed by the benefits of improved inflammatory control.
15. How do I know if topical calcineurin inhibitors are working, and when should I consider switching treatments?
You'll know topical calcineurin inhibitors are working when you observe several progressive improvements in your skin condition. Initially, you should notice a reduction in itching and burning sensations within the first few days to a week of consistent use. Visual improvements typically follow, with decreased redness, swelling, and scaling over 1-3 weeks. Skin texture should gradually improve, becoming smoother and less rough or leathery. Sleep disturbances related to itching should diminish, and the frequency and intensity of flares should reduce with continued treatment. Consider discussing treatment changes with your healthcare provider if you don't see noticeable improvement after 2-3 weeks of consistent use, if your symptoms worsen despite treatment, if you experience persistent uncomfortable application site reactions that don't improve, if you develop signs of skin infection at treatment sites, or if your condition initially improves but then plateaus below your treatment goals. Your provider might consider adjusting the concentration, changing formulations, combining with other treatments, or investigating potential triggers or complications that might be limiting treatment effectiveness.